
Therefore, most canonical amino acids in proteins can be exchanged with Ala by point mutations while the secondary structure remains intact. Dominant secondary structures in life as we know it are α-helices and β-sheets and most canonical amino acids can be regarded as chemical derivatives of Alanine. amino acids) for ribosomal protein synthesis is rather limited to those Alanine derivatives that are suitable for building α-helix or β-sheet secondary structural elements. In this model the selection of monomers (i.e.
#STRUCTURE OF AN AMINO ACID CODE#
This hypothesis explains the evolutionary choice of amino acids in the repertoire of the genetic code from a chemical point of view. On the basis of this fact the "Alanine World" hypothesis was proposed.
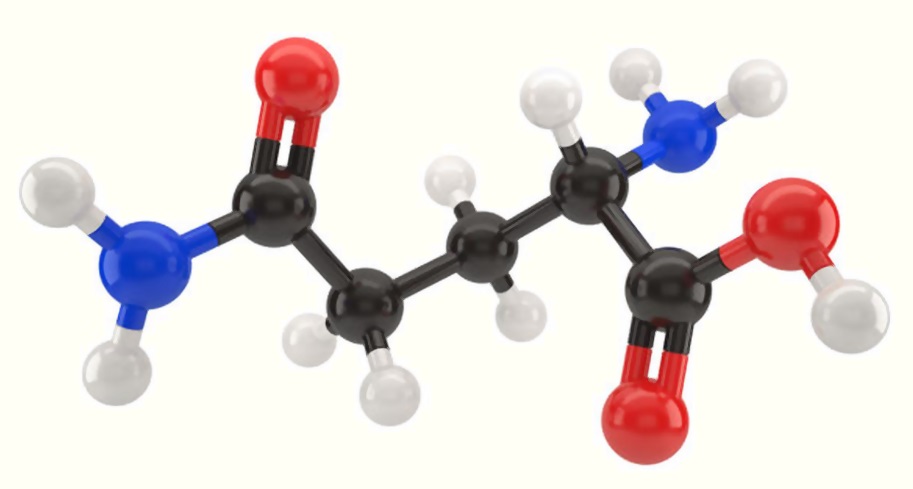
Alanine is believed to be one of the earliest amino acids to be included in the genetic code standard repertoire. : 721 Alanine World Hypothesis Īlanine is one of the twenty canonical α-amino acids used as building blocks (monomers) for the ribosome-mediated biosynthesis of proteins. The direction of the process is largely controlled by the relative concentration of the substrates and products of the reactions involved. Degradation Īlanine is broken down by oxidative deamination, the inverse reaction of the reductive amination reaction described above, catalyzed by the same enzymes. Racemic alanine can be prepared by the condensation of acetaldehyde with ammonium chloride in the presence of sodium cyanide by the Strecker reaction, or by the ammonolysis of 2-bromopropanoic acid. Fermentation routes to L-alanine are complicated by alanine racemase. L-Alanine is produced industrially by decarboxylation of L-aspartate by the action of aspartate 4-decarboxylase. : 721 Because transamination reactions are readily reversible and pyruvate is present in all cells, alanine can be easily formed and thus has close links to metabolic pathways such as glycolysis, gluconeogenesis, and the citric acid cycle. The net result is that pyruvate and ammonia are converted to alanine, consuming one reducing equivalent. In the second step, the amino group of the newly-formed glutamate is transferred to pyruvate by an aminotransferase enzyme, regenerating the α-ketoglutarate, and converting the pyruvate to alanine. In the first step, α-ketoglutarate, ammonia and NADH are converted by glutamate dehydrogenase to glutamate, NAD + and water. Alanine is found in a wide variety of foods, but is particularly concentrated in meats.Īlanine can be synthesized from pyruvate and branched chain amino acids such as valine, leucine, and isoleucine.Īlanine is produced by reductive amination of pyruvate, a two-step process. Alanine is a nonessential amino acid, meaning it can be manufactured by the human body, and does not need to be obtained through the diet. The methyl side-chain of alanine is non-reactive and is therefore hardly ever directly involved in protein function. Alanine is the simplest α-amino acid after glycine. The amino acid was named Alanin in German, in reference to aldehyde, with the interfix -an- for ease of pronunciation, the German ending -in used in chemical compounds being analogous to English -ine.Īlanine is an aliphatic amino acid, because the side-chain connected to the α-carbon atom is a methyl group (-CH 3). History and etymology Īlanine was first synthesized in 1850 when Adolph Strecker combined acetaldehyde and ammonia with hydrogen cyanide. The right-handed form, D-alanine, occurs in polypeptides in some bacterial cell walls : 131 and in some peptide antibiotics, and occurs in the tissues of many crustaceans and molluscs as an osmolyte. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The L- isomer of alanine ( left-handed) is the one that is incorporated into proteins.
It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH 3 +) and its carboxyl group deprotonated (as −CO 2 −). Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid.

It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Alanine (symbol Ala or A), or α-alanine, is an α- amino acid that is used in the biosynthesis of proteins.


 0 kommentar(er)
0 kommentar(er)
